Have you ever pulled an all-nighter and found yourself rummaging through the fridge the next day, craving sugary snacks or salty chips?
That is not just exhaustion talking—it is your hormones.
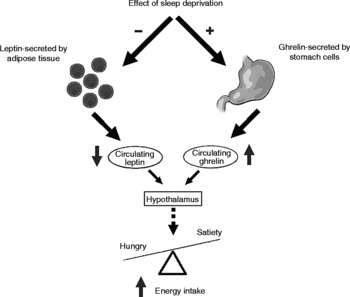
Specifically, ghrelin and leptin. These two hormones are responsible for regulating hunger and fullness, and they do not take kindly to a lack of sleep. Ghrelin encourages you to eat, while leptin tells you to stop.
But when you are sleep-deprived, this delicate hormonal balance is thrown off, triggering increased appetite and poor food choices.
In this article, Lean And Fit research team would explore how sleep deprivation affects ghrelin and leptin levels, using the latest scientific findings to explain why your late nights might be sabotaging your appetite control.
This Article Covers the Following Points:
- Sleep deprivation increases ghrelin and decreases leptin
- How sleep loss affects appetite hormones ghrelin and leptin
- Sleep deprivation and hormonal imbalance: ghrelin and leptin
- Sleep deprivation’s impact on hunger hormones
- Sleep deprivation and appetite-regulating hormones
- Sleep deprivation and ghrelin-leptin imbalance
- FAQs on Sleep Deprivation & Ghrelin and Leptin Levels
- Takeaway
Sleep Deprivation Increases Ghrelin and Decreases Leptin
Think about the last time you pulled an all-nighter or stayed up way past midnight binge-watching a show.
The next day, did you find yourself hungrier than usual—maybe reaching for a doughnut or a second helping of pasta?
That is not just lack of willpower talking; it is your hormones, especially ghrelin and leptin, going haywire thanks to poor sleep.
Studies have shown that when you skimp on sleep, ghrelin—the hormone that signals hunger—goes up, while leptin—the hormone that tells you you’re full—takes a nosedive.
In a well-known study published in Annals of Internal Medicine (Spiegel et al., 2004), participants who slept only four hours a night for two nights experienced an 18% drop in leptin and a 28% jump in ghrelin. Unsurprisingly, they reported being hungrier and craving high-carb foods like candy, bread, and pasta.
Another study from PLoS Medicine (Taheri et al., 2004) involving over 1,000 adults found that the less sleep people got, the higher their BMI—largely because of these same hormonal shifts.
And a 2022 study by Benedict and colleagues at Uppsala University confirmed that even one night of sleep deprivation was enough to knock ghrelin and leptin off balance. Leptin resistance and obesity is directly linked to one another.
Interestingly, women and individuals with higher body weight seemed especially sensitive to these changes.
So the next time you are tempted to sacrifice sleep for a late-night snack or one more episode, remember—your hormones are taking notes, and they’re voting for cookies.

How Sleep Loss affects Appetite Hormones Ghrelin and Leptin?
Normally, your hormones work like a well-rehearsed symphony.
Ghrelin rises to cue hunger before meals, then retreats after you eat. Meanwhile, leptin quietly whispers to your brain, “We are full—no need for seconds.”
During a good night’s sleep, leptin production ramps up and ghrelin drops, setting the stage for a balanced appetite the next day.
But when you shortchange sleep, this harmony turns into hormonal chaos. Leptin tanks, removing the brakes on hunger, while ghrelin spikes, flooring the gas pedal.
It is like your body yelling, “Feed me!” at full volume, even if you just had a meal. Suddenly, you are craving chocolate muffins at 9 a.m. and eyeing pizza for lunch—your body’s subtle sabotage in action.
Research published in Nature Communications shows that sleep-deprived brains light up in reward centers when exposed to high-calorie food, explaining why you would bypass a salad for fries without a second thought.
Add in mood dips and slower impulse control from poor sleep, and that vending machine snack starts looking like a gourmet meal.
In short: less sleep equals more hunger, worse choices, and a hormonal green light for overeating.
The hormonal chaos triggered by sleep deprivation is not just a bad day at the buffet—it can snowball into serious metabolic consequences.
Ghrelin, your hunger-stimulating hormone, becomes overactive when you are sleep-deprived, nudging you to eat more frequently and in larger quantities.
Leptin, meanwhile, which normally signals satiety to the brain, gets dialed down.
This one-two punch—more ghrelin, less leptin—creates a biological trap: you feel hungrier, stay hungry longer, and are less likely to stop eating when you should.
Over time, this hormonal mismatch encourages chronic overeating and steady weight gain, especially in the form of visceral fat—the dangerous kind that surrounds internal organs. But the ripple effects go further.
Disrupted leptin levels impair the hypothalamus’s role in regulating metabolism, while excess ghrelin has been shown to blunt insulin sensitivity.
According to studies from the Journal of Clinical Endocrinology & Metabolism and other endocrinology journals, people who sleep less than six hours a night not only eat more but also metabolize sugars less effectively.
This increases blood glucose levels and raises the risk for insulin resistance and metabolic dysregulation and type 2 diabetes. So, sleep is not just rest—it is a nightly hormonal reset your metabolism can’t afford to miss.
Sleep Deprivation’s Impact on Hunger Hormones
Chronic sleep deprivation does not just disrupt your schedule—it rewires your biology.
Night after night of insufficient rest locks your body into a hormonal tug-of-war that favors hunger and fat storage.
Ghrelin, the hormone that stimulates appetite, remains elevated, constantly signaling your brain to eat.
Meanwhile, leptin, which tells you when you are full, stays suppressed. This imbalance encourages frequent snacking, larger portion sizes, and a strong preference for high-calorie, sugary foods.
Over time, this creates a vicious cycle where the more you sleep poorly, the more your body demands energy it doesn’t need.
But it does not stop there. Sleep deprivation also spikes cortisol levels—your body’s primary stress hormone.
When cortisol is persistently elevated, it promotes abdominal fat storage and interferes with insulin sensitivity, making it harder for your body to regulate blood sugar effectively.
Studies from institutions like the University of Chicago and Harvard Medical School show that people who average less than six hours of sleep per night are significantly more likely to become overweight or obese.
The combination of high ghrelin, low leptin, and high cortisol creates a perfect storm for weight gain, metabolic slowdown, and appetite dysregulation—turning your body into a fat-storing machine rather than a fat-burning one.
Sleep Deprivation and Appetite-Regulating Hormones
The hormonal disruption from sleep deprivation does not stop at ghrelin and leptin—it sets off a chain reaction affecting multiple appetite-regulating systems.
When you sleep poorly, the body’s natural circadian rhythm becomes misaligned, which alters the timing and release of key hormones like insulin, cortisol, and peptide YY.
These changes make you feel hungry at odd hours—like craving pancakes at midnight—and can delay the feeling of fullness even after a complete meal.
For instance, research from the American Journal of Clinical Nutrition shows that just one night of inadequate sleep can reduce peptide YY, a hormone that helps suppress appetite after eating.
At the same time, elevated evening cortisol—common with chronic sleep loss—can enhance reward-seeking behavior, driving cravings for high-fat, high-sugar foods.
The effects are of one-size-fits-all. Studies suggest that women and individuals with higher body mass index (BMI) experience more significant disruptions in ghrelin and leptin balance after sleep loss.
These individuals may be biologically more sensitive to the appetite-amplifying effects of poor sleep, reinforcing the need for tailored interventions that account for gender, weight, and lifestyle factors.
Sleep is not just personal—it is hormonal strategy in action.
Sleep Deprivation and Ghrelin-Leptin Imbalance
A chronic imbalance between ghrelin and leptin does not just make you hungrier—it fundamentally shifts how your body handles energy.
Elevated ghrelin levels do not just stimulate appetite; they may also lower your resting energy expenditure, meaning your body burns fewer calories at rest.
At the same time, reduced leptin levels trick your brain into thinking you are in a state of starvation, even if you have just eaten.
This double signal urges your body to store more fat and conserve energy, the exact opposite of what you want if you are trying to lose weight.
Imagine someone trying to stick to a calorie-controlled diet after only five hours of sleep.
Despite eating a healthy breakfast, their ghrelin is telling them they are still starving, and their leptin isn’t signaling fullness.
Result?
They find themselves snacking on cookies by mid-morning—genuinely feeling hungry, not just “emotional eating.”
This is why modern weight loss plans increasingly include sleep hygiene—like setting consistent bedtimes or limiting blue light exposure—as essential strategies.
Without correcting sleep, no diet will work long-term.
Sleep is not a luxury; it is your body’s built-in appetite regulator, quietly running the show behind the scenes.
How Sleep Deprivation Alters Ghrelin and Leptin Levels?
The hormonal fallout from even a single night of bad sleep is not just “in your head”—it is in your bloodstream.
Research shows that one night of sleep deprivation can increase ghrelin (your hunger hormone) by nearly 15% and decrease leptin (your satiety hormone) by over 20%.
This shift leaves you hungrier than usual, craving comfort foods, and far less satisfied after meals. In other words, after a rough night, your body thinks it is time for a pizza buffet… for breakfast.
A study from the University of Chicago (Spiegel et al., 2004) revealed these exact changes after just two nights of four-hour sleep, causing participants to report intense cravings for calorie-dense foods.
Another study in Journal of Clinical Endocrinology & Metabolism found that these hormonal disruptions could impair insulin sensitivity and slow metabolic rate, compounding the problem.
These changes do not fade away—they build up.
Chronic sleep restriction leads to persistent ghrelin elevation and leptin suppression, making it harder to stop eating and easier to gain weight.
It is not just a willpower issue—it is a full-blown hormonal ambush.
Getting 7–9 hours of quality sleep is not a luxury; it is an appetite-regulating, metabolism-protecting, donut-deflecting necessity.
FAQs on Sleep Deprivation & Ghrelin and Leptin Levels
Q-1: Does sleeping less really change hunger hormones—or is it just willpower?
A-1: Consistently short sleep tends to lower leptin (the “I’m full” signal) and raise ghrelin (the “I’m hungry” signal). People report stronger cravings and larger portions after several nights of reduced sleep. Not everyone responds the same—sex, age, and starting weight matter—but the overall pattern favors increased appetite when sleep is cut back.
Q-2: Why do night shifts or irregular schedules worsen cravings even if I get enough total hours?
A-2: Timing matters as much as duration. Sleeping and eating at biologically “off” hours (circadian misalignment) can suppress leptin and disrupt the usual 24-hour rhythm of hunger and fullness. Even with seven to eight hours total, odd timing pushes you toward more frequent snacking and ultra-processed choices. Keeping light exposure, meal timing, and sleep/wake times consistent helps stabilize appetite signals.
Q-3: How fast do leptin and ghrelin change when I cut sleep—and do one-nighters matter?
A-3: Shifts can appear within a few days of partial sleep restriction, especially if it repeats across the workweek. A single all-nighter shows mixed effects—some people notice little change, others feel noticeably hungrier—but it’s chronic short sleep that most reliably resets appetite biology. If you rebound with regular, adequate sleep, hormones generally drift back toward baseline within days.
Q-4: Can improving sleep actually reduce calorie intake without a formal diet?
A-4: Yes. Extending nightly sleep by even 45–90 minutes often leads to fewer spontaneous snacks and lower intake of sugary foods, likely because leptin steadies and late-night eating cues fade. Pair sleep extension with earlier meal timing and a wind-down routine; many people find cravings ease and portion control feels more automatic—no strict rules required.
Q-5: What routine best keeps leptin steady and ghrelin in check when life is busy?
A-5: Aim for 7–9 hours in a regular window, anchor your first meal after morning light exposure, and finish the last substantial meal 2–3 hours before bed. If you work nights, consolidate sleep in one block, hold meal times steady across shifts, and use bright light at the start of your “day.” Add a protein-forward breakfast (or first meal) and limit large, late meals—these steps align sleep and feeding rhythms, supporting calmer hunger signals throughout the day.
Our Conclusive Analysis
Sleep deprivation throws a wrench into your body’s most fundamental regulatory systems. Ghrelin surges, urging you to eat more, while leptin drops, silencing the signal that tells you to stop.
This hormonal disruption does not just make you hungrier—it makes it harder to resist unhealthy food and easier to gain weight.
Over time, it sets the stage for obesity, insulin resistance and metabolic dysfunction.
The takeaway?
Sleep is not just for rest—it is a critical part of how your body controls hunger and energy use.
To manage your appetite, maintain a healthy weight, and support long-term metabolic health, start by getting enough quality sleep.
Your ghrelin and leptin levels will thank you.
References: