Abdominal obesity has emerged as a major public health concern worldwide.
This article explores how abdominal obesity worsens the risk of metabolic syndrome by delving into the scientific evidence that links excess visceral fat to metabolic dysregulation.
We will examine the underlying mechanisms, discuss the impact of inflammation and insulin resistance, and highlight both lifestyle and medical interventions.
Scientific studies and clinical research provide strong backing for these observations.
By the end of this article, you will have a comprehensive understanding of why abdominal obesity is so detrimental to metabolic health and what strategies can help mitigate its risks.
Article Index
- Introduction
- Understanding Abdominal Obesity
- Overview of Metabolic Syndrome
- The Link Between Abdominal Obesity and Metabolic Syndrome
- Underlying Mechanisms and Scientific Evidence
- The Role of Inflammation and Insulin Resistance
- Impact of Lifestyle Factors
- Prevention and Treatment Strategies
- FAQs on Abdominal Obesity and Metabolic Syndrome
- Emerging Research and Trends
- Conclusion
Understanding Abdominal Obesity
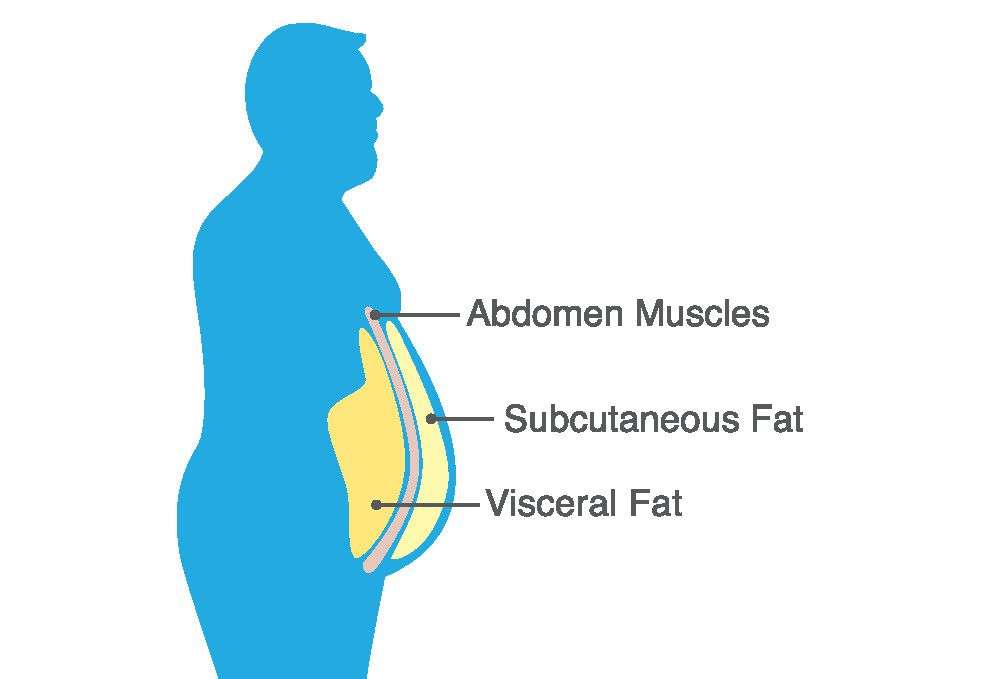
Abdominal obesity is defined by the excessive accumulation of fat around the stomach and abdomen, which is commonly measured by waist circumference.
Unlike subcutaneous fat, visceral fat is stored within the abdominal cavity and is highly metabolically active.
Research published in the Journal of Clinical Endocrinology & Metabolism has shown that visceral fat secretes hormones and inflammatory markers that can disrupt metabolic processes, contributing significantly to the development of metabolic syndrome.
This review of abdominal obesity metabolic syndrome risk factors helps underline why the location of fat is as important as the quantity.

Overview of Metabolic Syndrome
Metabolic syndrome is a cluster of conditions that together elevate the risk of cardiovascular diseases, stroke, and type 2 diabetes.
It typically includes high blood pressure, high blood sugar, abnormal cholesterol or triglyceride levels, and excess fat around the waist.
According to the American Heart Association, the coexistence of these conditions can lead to a synergistic increase in health risks, which makes early detection and intervention critical.
The interrelated nature of these risk factors makes it essential to understand how abdominal obesity acts as a central driver in this syndrome.
The Link Between Abdominal Obesity and Metabolic Syndrome
Multiple studies have established that abdominal obesity is closely linked to the onset of metabolic syndrome.
A landmark study published in Diabetes Care found that individuals with higher waist circumferences were more prone to developing insulin resistance, dyslipidemia, and hypertension.
This study underscored the fact that visceral fat, as opposed to peripheral fat, is more strongly correlated with metabolic complications.
Furthermore, the research into visceral fat metabolic syndrome research has shown that fat cells in the abdominal region release free fatty acids into the bloodstream, which interferes with the normal functioning of insulin and contributes to the overall metabolic dysregulation.
Underlying Mechanisms and Scientific Evidence
The mechanisms by which abdominal obesity worsens the risk of metabolic syndrome are multifactorial.
One primary pathway involves the secretion of adipokines—hormones produced by fat cells—that have both pro-inflammatory and anti-inflammatory properties. In individuals with excess abdominal fat, the balance shifts toward a pro-inflammatory state.
A study published in The Lancet Diabetes & Endocrinology provided evidence that increased levels of pro-inflammatory adipokines, such as tumor necrosis factor-alpha (TNF-α) and interleukin-6 (IL-6), are linked to the development of insulin resistance and cardiovascular diseases.
This scientific evidence supports the notion that chronic low-grade inflammation is a critical mediator in the progression of metabolic syndrome.
Another important mechanism is the hormonal dysregulation that occurs with excessive visceral fat. Abdominal obesity disrupts the normal balance of hormones like leptin and adiponectin, which regulate appetite and metabolism.
According to research in the Journal of Obesity, reduced adiponectin levels are associated with increased insulin resistance, while elevated leptin levels in obese individuals often indicate a state of leptin resistance. These hormonal disturbances create a vicious cycle where weight gain further disrupts metabolic homeostasis, making it increasingly difficult to reverse the effects once established.
The Role of Inflammation and Insulin Resistance
Inflammation and insulin resistance are at the heart of the metabolic disturbances caused by abdominal obesity.
The adipose tissue in the abdominal region is particularly prone to producing inflammatory cytokines. These cytokines contribute to systemic inflammation, which in turn impairs the body’s ability to respond to insulin.
A review article in Endocrine Reviews demonstrated that individuals with high levels of abdominal fat have elevated markers of inflammation, which are closely associated with the severity of insulin resistance.
Furthermore, the relationship between insulin resistance and abdominal obesity is well-documented. Insulin resistance, where cells fail to respond effectively to insulin, leads to higher circulating levels of glucose and insulin.
This condition not only predisposes individuals to type 2 diabetes but also exacerbates other components of metabolic syndrome, such as high blood pressure and dyslipidemia.
The keyword insulin resistance and abdominal obesity has been used in recent studies to focus on the metabolic consequences that arise from excess visceral fat accumulation.
Impact of Lifestyle Factors
Lifestyle choices are a major contributor to the development of both abdominal obesity and metabolic syndrome.
Diets high in refined sugars, saturated fats, and processed foods are known to promote visceral fat accumulation.
A study published in The American Journal of Clinical Nutrition found that high-calorie diets rich in processed foods significantly increase waist circumference and lead to metabolic derangements.
Conversely, adopting dietary interventions for abdominal obesity, such as increasing the intake of whole grains, fruits, and vegetables, has been shown to reduce visceral fat and improve metabolic outcomes.
Physical inactivity is another critical factor. Regular exercise has been proven to decrease visceral fat levels and improve insulin sensitivity.
The Journal of Applied Physiology highlights that both aerobic and resistance training can effectively reduce abdominal fat, thereby lowering the risk of metabolic syndrome.
Moreover, stress management and adequate sleep are essential, as chronic stress and sleep deprivation can disrupt hormonal balance and promote weight gain, particularly in the abdominal region.
Prevention and Treatment Strategies
Addressing abdominal obesity requires a multifaceted approach that integrates lifestyle modifications with medical interventions when necessary.
The first line of defense is often lifestyle changes for abdominal obesity, which include a balanced diet and regular physical activity.
Dietary interventions for abdominal obesity, as supported by clinical trials, emphasize the importance of reducing calorie intake and avoiding foods high in sugar and unhealthy fats.
Scientific studies have shown that even modest weight loss can lead to significant improvements in metabolic parameters.
Medical treatments for metabolic syndrome also play a role in managing the condition.
For individuals who do not respond adequately to lifestyle changes alone, medications that improve insulin sensitivity, such as metformin, or those that lower lipid levels, like statins, may be prescribed.
Furthermore, for individuals with severe obesity, bariatric surgery for visceral fat reduction has emerged as a viable option.
Research published in Obesity Surgery confirms that bariatric surgery not only results in significant weight loss but also improves or resolves many of the metabolic abnormalities associated with abdominal obesity.
Additionally, anti-inflammatory diets for metabolic syndrome have gained attention as a complementary approach.
Diets rich in omega-3 fatty acids, fiber, and antioxidants can reduce inflammation and improve metabolic outcomes.
Clinical trials have provided evidence that such diets can lower inflammatory markers and enhance insulin sensitivity, thereby reducing the overall risk of metabolic syndrome.
FAQs on Abdominal Obesity and Metabolic Syndrome
Q-1: How does abdominal obesity contribute to insulin resistance, a key component of metabolic syndrome?
A-1: Abdominal obesity, particularly the accumulation of visceral fat around internal organs, leads to insulin resistance by releasing free fatty acids into the bloodstream. These elevated fatty acid levels impair the body’s ability to respond to insulin, resulting in higher blood sugar levels and increased insulin production. Over time, this dysfunction can progress to type 2 diabetes.
Q-2: In what ways does abdominal obesity influence lipid metabolism, thereby increasing metabolic syndrome risk?
A-2: Excess abdominal fat disrupts lipid metabolism by elevating triglyceride levels and reducing high-density lipoprotein (HDL) cholesterol. This imbalance contributes to the development of atherogenic dyslipidemia, characterized by an increased number of small, dense low-density lipoprotein (LDL) particles. These lipid abnormalities promote plaque formation in arteries, heightening the risk of cardiovascular diseases associated with metabolic syndrome.
Q-3: How does abdominal obesity lead to chronic low-grade inflammation, and what impact does this have on metabolic syndrome?
A-3: Visceral fat acts as an active endocrine organ, secreting pro-inflammatory cytokines and adipokines. This secretion induces a state of chronic low-grade inflammation, which damages blood vessel linings and increases arterial stiffness. Such inflammatory processes elevate blood pressure and contribute to the development of metabolic syndrome.
Q-4: What is the relationship between abdominal obesity and hypertension in the context of metabolic syndrome?
A-4: Abdominal obesity contributes to hypertension through mechanisms such as increased sympathetic nervous system activity and elevated levels of pro-inflammatory cytokines. The accumulation of visceral fat leads to the release of substances that constrict blood vessels, resulting in higher blood pressure. This elevation in blood pressure is a significant component of metabolic syndrome.
Q-5: How does abdominal obesity affect the risk of developing non-alcoholic fatty liver disease (NAFLD), and what is its connection to metabolic syndrome?
A-5: Excess abdominal fat, especially visceral fat, increases the risk of NAFLD by promoting the accumulation of fat within liver cells. This hepatic fat buildup can lead to liver inflammation and fibrosis. NAFLD is closely associated with insulin resistance and is considered a hepatic manifestation of metabolic syndrome.
Q-6: Can abdominal obesity be present without other metabolic syndrome components, and what does this imply for health risk assessments?
A-6: While abdominal obesity is a central feature of metabolic syndrome, it can occur without other components such as hypertension or dyslipidemia. However, the presence of abdominal obesity alone still elevates the risk of developing cardiovascular diseases and type 2 diabetes. Therefore, monitoring abdominal fat distribution is crucial for early detection and prevention strategies, even in the absence of other metabolic abnormalities.
Emerging Research and Trends
The field of metabolic syndrome research is continuously evolving, with new studies offering fresh insights into the relationship between abdominal obesity and metabolic health.
Recent advances in imaging technologies, such as MRI and CT scans, have improved our ability to accurately measure visceral fat and assess its impact on metabolic risk.
These techniques have been instrumental in advancing our understanding of how fat distribution correlates with metabolic abnormalities.
Moreover, emerging trends in metabolic syndrome research are focused on personalized medicine.
Researchers are increasingly examining how genetic factors influence an individual’s susceptibility to the adverse effects of abdominal obesity.
For instance, genome-wide association studies (GWAS) have identified several genetic variants that predispose individuals to both abdominal obesity and metabolic syndrome.
This line of inquiry promises to yield tailored interventions that target the specific metabolic pathways affected in each patient.
The development of novel pharmacological agents is also an exciting area of progress. Several experimental drugs targeting the inflammatory and hormonal pathways involved in visceral fat accumulation are currently undergoing clinical trials.
These emerging trends in metabolic syndrome research underscore the potential for new therapies that could offer more effective and personalized treatment options for those at risk.
Conclusion
Abdominal obesity is a critical factor in the development and progression of metabolic syndrome, significantly increasing the risk of heart disease, stroke, and type 2 diabetes.
Scientific evidence from multiple high-quality studies supports the notion that excess visceral fat disrupts hormonal balance, promotes chronic inflammation, and impairs insulin sensitivity—all of which contribute to metabolic dysfunction.
We have examined how dietary interventions for abdominal obesity, combined with lifestyle changes and medical treatments, can help mitigate these risks.
The review of research demonstrates that addressing abdominal obesity requires a comprehensive strategy that includes both preventive measures and therapeutic interventions.
Incorporating lifestyle changes for abdominal obesity, such as improved nutrition and regular exercise, is essential for reducing visceral fat and improving metabolic health.
For those with more severe conditions, medical treatments for metabolic syndrome, including bariatric surgery for visceral fat reduction, offer promising outcomes.
In summary, the relationship between abdominal obesity and metabolic syndrome is complex and multifactorial.
Evidence supports that inflammation in metabolic syndrome, along with insulin resistance and hormonal imbalances, plays a significant role in the progression of the condition.
By adopting anti-inflammatory diets for metabolic syndrome and integrating emerging research findings, individuals can take proactive steps toward reducing their risk.
According to leanandfit.info, “staying informed about emerging trends in metabolic syndrome research is vital for both healthcare providers and patients, as personalized treatment options continue to evolve”.
Ultimately, the journey toward improved metabolic health begins with awareness, early intervention, and a commitment to making positive lifestyle changes.
References: