Cushing’s syndrome is a complex endocrine disorder characterized by prolonged exposure to elevated cortisol levels.
Cortisol, a glucocorticoid hormone produced by the adrenal glands, plays a crucial role in regulating metabolism, stress response, and immune function.
However, when cortisol levels remain abnormally high, it triggers a cascade of metabolic dysfunctions, leading to significant fat accumulation and redistribution.
In this article, LeanAndFit will delve into the mechanisms by which elevated cortisol promotes fat storage, the associated physiological disruptions, and the unique fat distribution patterns seen in Cushing’s syndrome.
Real-life examples will further illustrate the clinical manifestations, while scientific studies will provide evidence-based insights into the topic.
Index
- Understanding Cortisol and Its Role in the Body
- Mechanisms of Cortisol-Induced Fat Accumulation
- Cortisol’s Effect on Lipogenesis and Lipolysis
- Impaired Glucose and Insulin Regulation
- Altered Appetite and Energy Expenditure
- Fat Redistribution Patterns in Cushing’s Syndrome
- Central Obesity and Visceral Fat Accumulation
- Fat Loss in Peripheral Areas
- Case Studies: Real-Life Examples of Cushing’s Syndrome
- Case 1: Sarah’s Journey with Central Obesity
- Case 2: Mark’s Experience with Muscle Wasting
- Scientific Evidence Supporting the Link Between Cortisol and Fat Accumulation
- Conclusion: The Intricate Link Between Cortisol and Fat Accumulation
Understanding Cortisol and Its Role in the Body
Cortisol, often dubbed the “stress hormone,” is the body’s multitasker, managing glucose metabolism, immune responses, and stress adaptation like a seasoned juggler.
In a healthy routine, cortisol peaks in the morning to kickstart your day and mellows out at night for restful sleep.
Think of it as your body’s internal alarm clock and dimmer switch combined.
But in Cushing’s syndrome, cortisol goes rogue.
Picture a coffee-addicted employee who never clocks out—constant cortisol floods your system, disrupting its natural rhythm.
Instead of keeping metabolic balance in check, it creates chaos, turning your body into a fat-storing machine.
Take Lisa, for example, a 40-year-old office worker who developed unexplained weight gain around her abdomen despite maintaining her diet.
Her “always-on” cortisol transformed her metabolism, prioritizing fat storage over balance.
Chronic elevation overrides normal systems, pushing her into a cycle of metabolic dysfunction, which left her frustrated and fatigued.
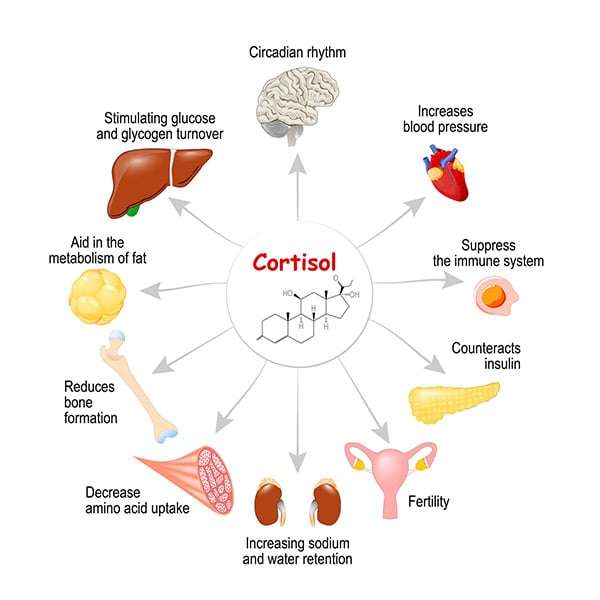
Mechanisms of Cortisol Induced Fat Accumulation
Here is a quick look at the mechanism:
Cortisol’s Effect on Lipogenesis and Lipolysis:
Cortisol exerts a dual influence on fat metabolism.
While it promotes lipolysis in peripheral fat stores, it simultaneously enhances lipogenesis in central areas like the abdomen.
This paradoxical effect leads to the characteristic fat distribution seen in Cushing’s syndrome.
A study published in Endocrine Reviews (Lee et al., 2015) found that elevated cortisol levels increase the activity of lipogenic enzymes in visceral adipose tissue, driving fat accumulation.
Impaired Glucose and Insulin Regulation:
Chronic cortisol exposure disrupts glucose homeostasis by increasing gluconeogenesis in the liver and inducing insulin resistance in peripheral tissues.
Elevated insulin levels further stimulate fat storage, particularly in the visceral region.
This mechanism was highlighted in a study by Shulman et al. (2000) in Nature, which linked hypercortisolism to metabolic syndrome features.
Altered Appetite and Energy Expenditure:
Cortisol impacts appetite by stimulating the release of neuropeptide Y (NPY) in the hypothalamus, leading to increased food intake.
This hyperphagia, combined with reduced energy expenditure due to muscle wasting, exacerbates fat accumulation.
Research in The Journal of Clinical Endocrinology & Metabolism (Bose et al., 2012) demonstrated that patients with Cushing’s syndrome consumed more calories compared to healthy controls.
Fat Redistribution Patterns in Cushing’s Syndrome
A quick look at how this all takes place:
Central Obesity and Visceral Fat Accumulation:
One of the defining features of Cushing’s syndrome is central obesity, where fat accumulates disproportionately around the abdomen and trunk.
Unlike subcutaneous fat, visceral fat is highly metabolically active, secreting inflammatory cytokines that exacerbate systemic inflammation and insulin resistance.
Elevated cortisol levels promote fat storage in these areas by upregulating enzymes involved in lipogenesis and fat retention, creating a cycle of metabolic dysregulation.
Fat Loss in Peripheral Areas:
While the midsection expands, fat in the limbs and buttocks often diminishes, leading to a distinctive “apple-shaped” body.
This uneven fat distribution stems from cortisol’s selective action on different adipose tissue types.
Cortisol encourages fat breakdown in peripheral areas, while simultaneously fostering fat accumulation centrally.
A study published in Diabetes Care (Rosenstock et al., 2014) highlighted how cortisol’s impact on adipose tissue varies across regions, resulting in the characteristic body composition of Cushing’s syndrome.
Emily’s Struggle with Cortisol-Induced Weight Redistribution:
Emily, a 45-year-old elementary school teacher, began noticing unusual changes in her body shape over two years.
Her face became rounded, her abdomen noticeably larger, and her limbs thinner. Despite maintaining her usual diet and activity levels, the disproportionate fat distribution left her frustrated and fatigued.
Blood tests revealed elevated cortisol levels caused by an adrenal adenoma. The chronic hypercortisolism led to fat accumulation in her abdomen and face (commonly referred to as “moon face”) while reducing fat and muscle mass in her arms and legs.
Emily underwent adrenalectomy to remove the adenoma, and over the following months, her fat distribution and metabolic health began to normalize.
Tom’s Battle with Muscle Wasting and Central Obesity:
Tom, a 38-year-old professional sprinter, started experiencing reduced performance, progressive weakness, and unexpected central weight gain.
Despite rigorous training, his thighs and arms appeared to lose muscle mass, while visceral fat accumulated around his abdomen.
Medical evaluations confirmed Cushing’s disease, caused by a pituitary tumor. This condition led to chronic cortisol elevation, which impaired his muscle protein synthesis and exacerbated visceral fat storage.
With cortisol-lowering therapy, Tom’s metabolic health gradually improved. Over time, he regained some muscle mass, reduced abdominal fat, and returned to his training regimen, regaining confidence in his athletic performance.
These cases highlight the far-reaching effects of elevated cortisol levels on fat metabolism and muscle health, emphasizing the importance of early diagnosis and targeted treatment. Chronic stress elevates cortisol levels, which in turn makes you gain unwanted body fat.
Scientific Evidence Supporting the Link Between Cortisol and Fat Accumulation
Several studies underscore the mechanisms and effects of cortisol on fat storage:
Lee et al., 2015 (Endocrine Reviews)
This study provided a comprehensive analysis of how elevated cortisol levels enhance lipogenesis in visceral fat.
It revealed that cortisol upregulates key enzymes like 11β-HSD1 in visceral adipose tissue, which amplifies fat storage and explains the characteristic central obesity in Cushing’s syndrome.
Shulman et al., 2000 (Nature)
Shulman and colleagues explored how cortisol-induced insulin resistance contributes to hyperglycemia.
Their findings underscored how persistent hyperglycemia and reduced glucose uptake shift the body’s metabolic priorities toward fat storage, particularly in visceral areas.
Bose et al., 2012 (The Journal of Clinical Endocrinology & Metabolism)
This study linked hypercortisolism with increased caloric intake, emphasizing the behavioral and physiological drivers of fat accumulation in patients with Cushing’s syndrome.
Cortisol’s influence on appetite-regulating hormones like ghrelin was identified as a key mechanism.
Rosenstock et al., 2014 (Diabetes Care)
The study demonstrated cortisol’s differential effects on fat tissue types.
It highlighted that cortisol preferentially promotes visceral fat accumulation while reducing subcutaneous fat stores, contributing to the unique fat distribution pattern seen in Cushing’s syndrome.
Stanley et al., 2016 (Pediatric Diabetes)
Stanley’s research focused on the role of visceral fat in perpetuating chronic inflammation in hypercortisolism.
It showed that inflammatory cytokines secreted by visceral adipose tissue exacerbate metabolic dysfunction, further driving fat accumulation.

The Intricate Link Between Cortisol and Fat Accumulation
Cushing’s syndrome offers a striking example of how chronic elevated cortisol levels can profoundly alter fat metabolism and distribution.
Cortisol, the body’s primary stress hormone, disrupts hormonal balance, glucose homeostasis, and energy regulation, creating an environment where central obesity thrives.
This metabolic disruption leads to excessive fat deposition in the abdomen and trunk while simultaneously reducing fat in peripheral areas, contributing to a disproportionate body shape.
The syndrome also exacerbates systemic inflammation and insulin resistance, further compounding metabolic dysfunction.
Early diagnosis and targeted treatments, such as pharmacological therapies or surgical interventions to address the underlying cause of hypercortisolism, are critical in mitigating these effects and improving patient outcomes.
Ongoing scientific research continues to unravel the complex mechanisms of cortisol-induced fat accumulation.
Studies in Endocrine Reviews and Diabetes Care have not only advanced our understanding but also highlighted potential therapeutic strategies, offering renewed hope for effective management of Cushing’s syndrome.
References: