Insulin resistance is a multifaceted metabolic disorder that significantly contributes to weight gain and fat accumulation.
Under normal circumstances, insulin acts as a key, allowing glucose to enter cells for energy or storage.
However, in insulin resistance, cells fail to respond efficiently to insulin’s signals, causing glucose to remain in the bloodstream.
This triggers the pancreas to produce more insulin, leading to hyperinsulinemia.
Elevated insulin levels create a metabolic environment favoring fat storage over fat utilization.
Insulin promotes lipogenesis, the process of converting glucose into fat, while simultaneously inhibiting lipolysis, the breakdown of fat for energy.
This dual action not only drives the buildup of fat, especially around visceral regions, but also exacerbates insulin resistance, creating a vicious cycle.
This article delves into the mechanisms linking insulin resistance to fat storage, examining the physiological processes at play and the long-term implications for individuals.
By incorporating scientific insights and real-world examples, we aim to provide a comprehensive understanding of how insulin resistance fuels fat accumulation and contributes to metabolic challenges like obesity and Type 2 diabetes.
Table of Contents
- Introduction to Insulin Resistance and Fat Storage
- Mechanisms Linking Insulin Resistance to Fat Accumulation
- 2.1. Disrupted Glucose Uptake and Fat Storage
- 2.2. Elevated Insulin Levels and Lipogenesis
- 2.3. Impaired Hormonal Regulation of Fat Metabolism
- The Role of Visceral Fat in Insulin Resistance
- 3.1. Adipose Tissue Inflammation
- 3.2. Hormonal Dysregulation and Insulin Sensitivity
- Real-Life Case Studies: The Impact of Insulin Resistance on Fat Storage
- 4.1. Case Study: Sarah’s Struggle with Weight Gain and Fatigue
- 4.2. Case Study: John’s Path to Understanding Insulin Resistance
- FAQs on Insulin Resistance & Fat Storage
- Conclusion
Introduction to Insulin Resistance and Fat Storage
Insulin is a vital hormone that plays a key role in glucose regulation and energy balance.
Under normal conditions, insulin enables glucose to enter cells, where it is either used immediately for energy or stored as glycogen in the liver and muscles for future use.
This tightly regulated system ensures stable blood sugar levels and a balanced energy supply.
However, in individuals with insulin resistance, the cells’ response to insulin becomes impaired, meaning glucose remains in the bloodstream instead of being absorbed.
To compensate, the pancreas produces more insulin, leading to hyperinsulinemia.
While this helps reduce blood sugar temporarily, the persistently high insulin levels set off a cascade of metabolic effects that profoundly impact fat storage.
High insulin levels promote lipogenesis, or the conversion of glucose into fat, particularly in visceral fat stores. Simultaneously, insulin inhibits lipolysis, the breakdown of stored fat into fatty acids for energy.
This creates a metabolic environment favoring fat accumulation over fat utilization, leading to weight gain and further exacerbating insulin resistance.
This article delves into the intricate mechanisms by which insulin resistance drives fat storage, examining the role of hormonal imbalances, disrupted energy metabolism, and inflammation.
Supported by scientific studies and real-life case examples, it sheds light on how this metabolic dysfunction contributes to widespread health challenges such as obesity and diabetes.
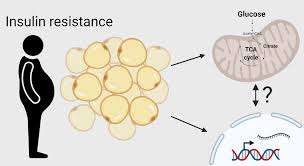
Mechanisms Linking Insulin Resistance to Fat Accumulation
Let us walk you through this process in brief:
Disrupted Glucose Uptake and Fat Storage:
In insulin-resistant individuals, glucose uptake by muscle and liver cells is impaired. The excess glucose in the bloodstream signals the pancreas to produce more insulin.
Elevated insulin levels drive glucose into adipocytes (fat cells), where it is converted into triglycerides for storage.
A study published in Diabetes (Saltiel & Kahn, 2001) highlighted how insulin resistance skews energy metabolism towards fat storage, exacerbating weight gain.
This process creates a vicious cycle, as increased fat storage further worsens insulin sensitivity.
Elevated Insulin Levels and Lipogenesis:
Insulin is a potent anabolic hormone that stimulates lipogenesis by activating enzymes such as acetyl-CoA carboxylase (ACC) and fatty acid synthase (FAS).
In insulin-resistant states, chronically high insulin levels enhance fat production, particularly in the liver and visceral fat stores.
This phenomenon explains why individuals with insulin resistance often develop non-alcoholic fatty liver disease (NAFLD), as excess glucose and fatty acids are stored in liver cells.
Research in The Journal of Clinical Investigation (Samuel & Shulman, 2012) demonstrated the link between hyperinsulinemia and enhanced lipogenesis in insulin-resistant subjects.
Impaired Hormonal Regulation of Fat Metabolism:
Insulin inhibits lipolysis, the breakdown of stored fat into free fatty acids. In insulin resistance, elevated insulin levels suppress fat breakdown even when energy demands are high.
This leaves individuals reliant on glucose for energy, perpetuating the cycle of fat storage and glucose accumulation.
Moreover, insulin resistance disrupts the balance of hormones such as leptin and adiponectin, which regulate appetite and fat metabolism.
A study in Endocrine Reviews (Rosen & Spiegelman, 2006) noted that decreased adiponectin levels in insulin resistance impair the body’s ability to burn fat effectively.
The Role of Visceral Fat in Insulin Resistance
Here is what LeanAndFit research team found:
Adipose Tissue Inflammation:
Visceral fat is metabolically active and prone to inflammation.
In insulin resistance, visceral fat cells release pro-inflammatory cytokines such as TNF-alpha and IL-6, which impair insulin signaling pathways.
This inflammation creates a feedback loop where increased visceral fat worsens insulin resistance, driving further fat accumulation.
A study in The Lancet Diabetes & Endocrinology (Després, 2012) emphasized that visceral fat is a stronger predictor of insulin resistance than total body fat, underscoring its unique role in metabolic dysfunction.
Hormonal Dysregulation and Insulin Sensitivity:
Visceral fat secretes hormones like resistin and leptin, which influence insulin sensitivity.
In insulin resistance, elevated leptin levels (leptin resistance) fail to suppress appetite, leading to overeating and additional fat storage.
Simultaneously, resistin impairs insulin signaling in muscle and liver cells, further exacerbating glucose and fat dysregulation.
This hormonal imbalance highlights the interconnectedness of insulin resistance and fat storage, making visceral fat a key target for intervention.
Impact of Insulin Resistance on Fat Storage on Sarah & John
Here are two classic cases of how extra body fat affects your natural insulin levels:
Case Study: Sarah’s Struggle with Weight Gain and Fatigue:
Sarah, a 42-year-old nurse with a demanding job, started noticing gradual weight gain despite sticking to her usual calorie intake.
She constantly felt fatigued, struggled with sugar cravings, and found herself unable to lose weight no matter how many diets she tried.
Concerned about her symptoms, Sarah sought medical advice. Blood tests revealed elevated fasting insulin levels and impaired glucose tolerance, both strong indicators of insulin resistance.
Sarah’s endocrinologist explained that her body’s inability to efficiently use insulin was causing excess glucose to circulate in her bloodstream.
This triggered her pancreas to produce more insulin, driving the glucose into fat cells instead of muscles for energy.
The result was increased fat storage, particularly around her abdomen, a hallmark of visceral fat accumulation linked to insulin resistance.
To address the issue, Sarah began a structured plan focused on low-glycemic foods that stabilized her blood sugar and reduced her body’s insulin demand.
She incorporated regular aerobic exercises and resistance training (such as dumbbell lunges), which helped her muscles use glucose more effectively and improved her insulin sensitivity.
Within six months, Sarah noticed significant reductions in her abdominal fat and a marked improvement in her energy levels, breaking the vicious cycle of fat accumulation and fatigue.
Case Study: John’s Path to Understanding Insulin Resistance:
John, a 50-year-old accountant, had struggled with obesity for years, recently culminating in a diagnosis of Type 2 diabetes.
He had long assumed his weight gain was due to a sedentary lifestyle and poor dietary choices. However, his doctor revealed that prolonged insulin resistance was a key factor contributing to his condition.
The high levels of insulin in John’s body not only promoted excess fat storage but also suppressed his body’s ability to burn fat for energy.
This reliance on glucose as a primary energy source left him feeling tired and exacerbated his weight gain, particularly around his abdomen.
Additionally, his visceral fat was releasing inflammatory markers, further impairing his metabolic health and worsening his insulin resistance.
Under medical supervision, John adopted a tailored exercise program that included weight training and high-intensity interval training (HIIT) to improve insulin sensitivity.
He also transitioned to a balanced diet rich in whole foods and lean proteins, cutting out processed sugars and refined carbs.
Over a year, John lost 25 pounds, significantly reduced his visceral fat, and brought his blood sugar levels under control.
His journey underscored the reversible aspects of insulin resistance when tackled with a combination of lifestyle changes and medical guidance.
FAQs on Insulin Resistance & Fat Storage
Q-1: If insulin “doesn’t work,” why does insulin resistance still lead to more fat storage?
A-1: Insulin resistance is pathway-specific. In liver and muscle, insulin’s glucose-lowering actions are blunted, but in adipose tissue, several storage signals remain relatively intact. The pancreas compensates with higher insulin levels, which still activate fat-storage machinery (like lipoprotein lipase at fat cells and lipogenesis genes in liver). Net result: glucose isn’t burned efficiently in muscle, yet fat storage in adipose and liver keeps getting a green light.
Q-2: How does insulin resistance change where calories go right after a meal?
A-2: In healthy muscle, insulin rapidly shuttles glucose into cells for use or glycogen storage. With muscle insulin resistance, that “sink” shrinks. Glucose and dietary fat are redirected toward the liver and adipose tissue. The liver converts surplus carbohydrate to triglycerides (de novo lipogenesis) and exports them as VLDL; adipose tissue—primed by elevated insulin—clears those triglycerides and locks them into fat cells.
Q-3: What’s the role of elevated insulin (hyperinsulinemia) in expanding fat cells?
A-3: Because muscles and liver respond poorly, the body secretes more insulin to keep glucose in range. High insulin still suppresses fat breakdown enzymes and boosts fat-uptake enzymes around adipocytes. Over weeks to months, this favors triglyceride entry over exit, causing fat cells to swell (hypertrophy). Enlarged adipocytes then leak inflammatory signals that further worsen insulin resistance, creating a self-reinforcing loop.
Q-4: Does insulin resistance affect fat breakdown between meals, too?
A-4: Yes—basal insulin normally restrains excessive fat release from adipose tissue. In insulin-resistant fat, that restraint is blunted, so more fatty acids trickle into the bloodstream and to the liver. It has been proven that BPA in plastics leads to insulin resistance. The liver re-packages them into triglycerides, raising VLDL and providing yet more substrate for adipose storage later. Ironically, the same physiology that leaks fat between meals can still favor net storage across the day when post-meal insulin surges occur.
Q-5: How do microcirculation and mitochondria tie insulin resistance to fat gain?
A-5: Insulin normally opens capillary beds in muscle to deliver glucose; resistance reduces this “microvascular recruitment,” limiting fuel delivery where it would be burned. At the same time, mitochondrial carb oxidation in resistant muscle is sluggish, so the body relies more on liver fat-making and adipose storage to park energy safely. Less delivery + poorer burning = more overflow to storage depots. This in turn causes obesity induced hyperinsulinemia.
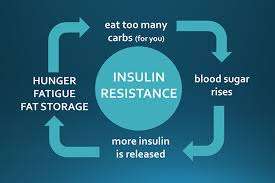
Q-6: What day-to-day pattern shows insulin resistance pushing storage over burning—and how can you disrupt it quickly?
A-6: The pattern is high-carb or high-fat meals → muted muscle uptake → bigger insulin waves → more liver triglyceride production and adipose uptake. Three fast disruptors: (1) a 10–15-minute walk after meals to divert glucose into muscle without needing much insulin; (2) higher-protein, higher-fiber meals to flatten post-meal insulin; (3) brief resistance work (even bodyweight) most days to restore muscle glucose uptake and shrink the overflow to fat.
Conclusion
Insulin resistance plays a pivotal role in increasing fat storage through mechanisms such as impaired glucose uptake, elevated insulin levels, and disrupted hormonal regulation.
The condition not only promotes lipogenesis but also suppresses lipolysis, creating a metabolic environment that favors fat accumulation, particularly in visceral fat stores.
Scientific studies and real-life cases illustrate the profound impact of insulin resistance on metabolic health, emphasizing the importance of early intervention.
By understanding the mechanisms behind insulin resistance and fat storage, individuals and healthcare providers can develop targeted strategies to mitigate its effects, reducing the risk of obesity and related metabolic disorders.
References: