When most people think about obesity, they picture an expanding waistline and maybe the challenges of squeezing into old jeans.
What often goes unnoticed is the metabolic chaos that obesity triggers beneath the surface.
One of its most significant effects?
Insulin resistance—a major driver of metabolic syndrome.
In this article, LeanAndFit research team shall shall dive deep into the fascinating (and sometimes frustrating) relationship between obesity and insulin resistance.
Here is what we shall cover:
- The biology behind insulin resistance and its connection to obesity.
- How fat distribution plays a critical role in metabolic dysfunction.
- Real-life examples of how obesity impacts insulin sensitivity.
- Scientific studies that shine a light on the obesity-insulin resistance connection.
- The complex relationship between central obesity, BMI, and metabolic syndrome.
So, buckle up—let’s unravel how extra pounds can lead to insulin resistance and wreak havoc on your health.
Article Contents:
- What is Insulin Resistance? A Quick Primer
- Understanding Metabolic Syndrome and Obesity
- How Fat Distribution Influences Insulin Resistance
- The Role of Inflammation in Obesity-Induced Insulin Resistance
- Abdominal Obesity: A Key Culprit in Metabolic Syndrome
- Scientific Evidence Linking Obesity and Insulin Resistance
- Real-Life Examples of the Obesity-Insulin Resistance Connection
- FAQs on Obesity & Insulin Resistance in Metabolic Syndrome
- The Long-Term Impact of Insulin Resistance in Metabolic Syndrome
What is Insulin Resistance? A Quick Primer
Imagine Sarah, a 35-year-old marketing professional.
Sarah’s long hours at a desk, coupled with her love for sugary snacks, have slowly added inches to her waistline. Over time, her body begins to change on a deeper, invisible level.
Insulin, the hormone responsible for regulating her blood sugar, is trying its best to maintain balance by signaling her cells to absorb glucose for energy.
However, Sarah’s excess fat—especially the visceral fat around her abdomen—begins to release harmful inflammatory molecules.
These molecules disrupt the normal function of her insulin receptors, akin to her body pressing “mute” on insulin’s instructions.
Despite insulin shouting its message to the cells, they do not respond effectively. As a result, glucose starts to pile up in her bloodstream.
This is the beginning of insulin resistance, where her cells become less sensitive to insulin, forcing her body to produce more of it just to keep up.
Over time, this cycle leads to chronically high blood sugar levels, setting the stage for metabolic syndrome and, eventually, type 2 diabetes.
In Sarah’s case, her obesity acts as the loudest mute button, blocking insulin’s crucial role in keeping her blood sugar levels in check.
This metabolic disruption now demands attention before it progresses further.
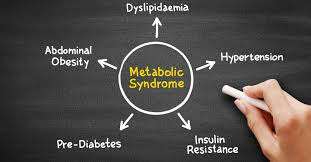
The Definition and Prevalence of Obesity and Metabolic Syndrome
Metabolic syndrome is a cluster of conditions—high blood pressure, elevated blood sugar, abnormal cholesterol levels, and, yes, abdominal obesity.
Together, these factors increase the risk of heart disease, stroke, and diabetes.
According to the World Health Organization, obesity is the most significant driver of metabolic syndrome, with central obesity playing a starring role.
A high body mass index (BMI), often used to define obesity, correlates strongly with the prevalence of metabolic syndrome.
But here is the catch: not all fat is created equal, and where you store it matters more than how much you have.
How Fat Distribution Influences Insulin Resistance?
Here is how your extra body fat tends to disrupt your body’s natural capability to handle insulin:
Central Obesity and Metabolic Syndrome
If you carry your weight in your midsection (hello, central obesity), your risk of insulin resistance skyrockets.
Abdominal fat is not just padding—it’s biologically active.
Visceral fat, the fat surrounding your organs, releases hormones and inflammatory molecules that interfere with insulin’s effectiveness.
Example:
Jane, a 45-year-old office worker with a sedentary lifestyle, noticed her waistline expanding over the years.
Despite normal BMI, her doctor diagnosed her with abdominal obesity and insulin resistance. She developed hyperinsulinemia as she was overweight.
It turns out that her visceral fat was silently driving her risk of metabolic syndrome.
The Role of Inflammation in Obesity-Induced Insulin Resistance
Obesity does not just mean extra weight—it transforms the body into a pro-inflammatory environment, triggering a cascade of metabolic disruptions.
Fat tissue, particularly visceral fat, is not just a passive storage depot; it’s an active endocrine organ that secretes inflammatory molecules known as cytokines, including tumor necrosis factor-alpha (TNF-alpha) and interleukin-6 (IL-6).
These cytokines interfere with insulin signaling by impairing the insulin receptor’s ability to function effectively, a process often referred to as “insulin resistance.”
This chronic inflammatory state does not operate in isolation.
It is frequently accompanied by oxidative stress—an imbalance between harmful free radicals and the body’s ability to neutralize them.
Together, inflammation and oxidative stress damage cells, reduce their sensitivity to insulin, and impair glucose uptake, leading to consistently elevated blood sugar levels.
A study published in Nature Reviews Endocrinology (2020) provided compelling evidence of this connection.
Researchers found that obese individuals with high levels of inflammatory markers were twice as likely to develop insulin resistance compared to those with lower inflammation.
This finding underscores the critical role inflammation plays as a bridge between obesity and metabolic dysfunction, making it a primary target for preventing the progression to type 2 diabetes and other complications associated with insulin resistance.
Abdominal Obesity: A Key Culprit in Metabolic Syndrome
Your waistline is not just a fashion concern; it’s a metabolic marker.
Research from the Journal of Clinical Endocrinology & Metabolism found that individuals with abdominal obesity were at a significantly higher risk for obesity and insulin resistance, even if their overall weight was normal.
This is why waist circumference, rather than BMI, is often a better predictor of metabolic syndrome.
Fat around the belly releases free fatty acids into the bloodstream, impairing insulin’s ability to do its job.
Example:
Mark, a 38-year-old gym enthusiast, always believed he was healthy because his BMI was in the normal range.
However, his abdominal obesity and metabolic syndrome diagnosis shocked him. So, his metabolic syndrome was super activated.
Despite his workout routine, his diet loaded with processed foods led to visceral fat accumulation.
Scientific Evidence Linking Obesity and Insulin Resistance
Numerous scientific studies have firmly established the connection between obesity and insulin resistance, shedding light on the underlying mechanisms at play:
- “Obesity and Insulin Resistance: The Role of Adipose Tissue” (The Lancet, 2021): This groundbreaking study examined how the expansion of fat cells alters their hormonal and inflammatory profiles, ultimately impairing insulin sensitivity. The findings highlighted how excess fat becomes metabolically active, releasing pro-inflammatory cytokines that disrupt insulin signaling pathways.
- “Impact of Central Obesity on Glucose Metabolism” (Diabetes Care, 2020): This research zeroed in on visceral fat—commonly found in the abdominal region—and its unique impact on glucose metabolism. The study revealed that visceral fat not only releases harmful free fatty acids but also interferes with glucose uptake in cells, resulting in elevated fasting blood sugar levels.
- “Adipokines and Their Role in Metabolic Syndrome” (Journal of Diabetes Research, 2019): Researchers explored the role of adipokines, hormones secreted by fat tissue, in the development of insulin resistance. The study emphasized imbalances in leptin (responsible for regulating appetite) and adiponectin (key to improving insulin sensitivity) as critical factors linking obesity to metabolic syndrome.
These studies collectively demonstrate how excess fat tissue, particularly in the abdominal region, initiates a cascade of metabolic disturbances that directly impair the body’s ability to regulate insulin and blood sugar effectively.
Real-Life Examples of the Obesity-Insulin Resistance Connection
A quick look at two such examples:
Meet Linda:
Linda, a 52-year-old teacher, struggled with her weight for years.
She was recently diagnosed with diabetes metabolic syndrome and obesity.
Her doctor explained how her chronic insulin resistance was linked to her weight gain, particularly around her midsection.
With lifestyle changes, Linda managed to improve her condition, but the journey was not easy.
Meet Jake:
Jake, a 60-year-old retiree, had always brushed off his expanding waistline as a natural part of “normal aging,” never suspecting it could signal deeper health concerns.
However, during a routine check-up, his doctor diagnosed him with central obesity and metabolic syndrome.
Jake was shocked to learn that the visceral fat accumulating around his abdomen was not just cosmetic—it was actively driving insulin resistance, disrupting his blood sugar regulation, and putting him at higher risk for type 2 diabetes.
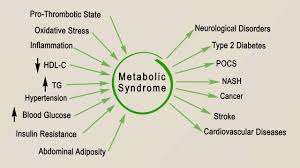
The Long-Term Impact of Insulin Resistance in Metabolic Syndrome
Unchecked insulin resistance is not a static condition—it is a progressive, evolving issue that intensifies over time.
Left unmanaged, it often escalates into more severe health complications, including full-blown type 2 diabetes, cardiovascular diseases, and non-alcoholic fatty liver disease (NAFLD).
These conditions do not just appear in isolation; they are interconnected and feed off each other, creating a cascade of metabolic dysfunction that becomes increasingly difficult to reverse.
Obesity significantly accelerates this decline by perpetuating a vicious cycle.
Excess fat, particularly visceral fat, amplifies inflammation, hormonal imbalances, and oxidative stress—three key drivers of insulin resistance.
This constant state of metabolic disruption strains the body’s ability to regulate glucose, lipids, and blood pressure, laying the groundwork for chronic diseases.
According to a report published in Diabetes Care, individuals with insulin resistance overweight face a staggering 40% higher risk of developing coronary artery disease compared to those with normal insulin sensitivity.
This alarming statistic underscores the urgent need for timely intervention.
Early diagnosis and management of insulin resistance—through lifestyle changes, weight management, and medical treatment—are crucial not only to halt its progression but also to reduce the risk of life-threatening complications like heart attacks, strokes, and liver failure.
FAQs on Obesity & Insulin Resistance in Metabolic Syndrome
Q-1: How does “adipose expandability” explain why obesity flips into insulin resistance?
A-1: Fat tissue can safely store only so much. Once that capacity is exceeded, spillover fat accumulates in liver and muscle as lipid intermediates (like DAGs and ceramides). These molecules interfere with insulin-signaling steps that normally move glucose into cells. A telltale pattern is “normal” or mildly elevated fasting glucose with high fasting insulin and rising triglycerides—early signs that storage has overflowed and tissues are resisting insulin’s signal.
Q-2: What role does immune activity inside fat play in metabolic syndrome?
A-2: As fat cells enlarge, oxygen delivery lags and tiny areas become stressed. Immune cells shift toward a pro-inflammatory profile and release cytokines that blunt insulin signaling locally and systemically. The result is a hormonal “noise” that keeps the liver making glucose and the muscle ignoring insulin. Practical flags include a growing waist circumference, low HDL with high triglycerides, and elevated liver enzymes even before glucose tests turn abnormal.
Q-3: Are hormones from fat itself part of the problem—or the solution?
A-3: Both. Healthy fat secretes adiponectin, which improves insulin sensitivity; with obesity, adiponectin falls while leptin rises—but the brain often becomes less responsive to leptin’s satiety message. That combo encourages higher appetite, less fat burning, and stiffer blood vessels. People often notice stronger cravings, easier weight regain after diets, and morning fatigue—behavioral clues that adipokine balance has shifted away from insulin sensitivity.
Q-4: How do cellular stress and mitochondria pull insulin off track?
A-4: Overloaded cells divert resources to damage control—managing oxidative and endoplasmic reticulum stress—while dialing back energy-hungry processes. Mitochondria handle fuel less efficiently, and stress pathways activate enzymes that block insulin’s downstream signals. In daily life, this shows up as lower exercise capacity, slower recovery from meals (longer glucose “tail”), and a need for more coffee or snacks to maintain focus.
Q-5: Why do liver and muscle “talk” each other into worse insulin resistance—and what amplifies it?
A-5: Visceral fat drains into the portal vein, bathing the liver in fatty acids that boost glucose production and raise VLDL triglycerides. Those lipids then deposit in muscle, worsening insulin resistance there—a two-way loop. Gut factors (less fiber diversity, more permeability) and circadian drift (late eating, poor sleep) amplify the loop. Simple counters: increase fiber variety, front-load calories earlier in the day, add brief post-meal walks, and do 2–3 weekly strength sessions to expand healthy storage and improve insulin response.
Bottom line: In metabolic syndrome, insulin resistance emerges from overfilled fat depots, inflammatory signaling, hormone imbalance, and organ cross-talk. Track waist size, triglyceride/HDL ratio, and fasting insulin alongside glucose—and use diet quality, movement, strength, and sleep timing to push the system back toward insulin sensitivity.
Unmasking the Connection Between Obesity and Insulin Resistance
The connection between obesity and insulin resistance is undeniable, with metabolic syndrome serving as the bridge that links the two.
Abdominal fat plays a critical role, actively disrupting glucose metabolism, while chronic inflammation further impairs insulin signaling.
Together, these factors create a cycle of metabolic dysfunction that, if left unchecked, can lead to serious complications like type 2 diabetes and cardiovascular disease.
Recognizing how and why obesity triggers insulin resistance is essential for breaking this cycle.
Awareness is the first step—whether you are navigating your own health journey or helping someone else.
By understanding the risks and mechanisms involved, individuals can make informed decisions about diet, exercise, and lifestyle changes to prevent further progression.
While the path may seem challenging, knowing the science behind obesity and insulin resistance empowers us to take control of our health and prioritize proactive solutions.
References: