As someone who has battled with weight gain for years, I always wondered why shedding those extra pounds seemed so impossible.
It turns out, the answer might lie deeper than just diet and exercise.
Obesity and hyperinsulinemia, a condition where the body produces too much insulin, are tightly linked.
You have probably heard about insulin in the context of diabetes, but did you know that hyperinsulinemia can exist without diabetes?
And, more importantly, obesity plays a direct role in causing this condition.
In this article, leanandfit.info would explore how obesity causes hyperinsulinemia, unraveling the mechanisms that tie these two conditions together.
What’s in This Article?
- What is Hyperinsulinemia?
- Understanding the Role of Insulin in the Body
- How Obesity Leads to Hyperinsulinemia
- The Vicious Cycle: Hyperinsulinemia and Weight Gain
- Scientific Evidence Linking Obesity and Hyperinsulinemia
- FAQs on Obesity & Hyperinsulinemia
- Daily Lifestyle Habits That Contribute to Hyperinsulinemia
- Conclusion: How Obesity Fuels the Fire of Hyperinsulinemia
What is Hyperinsulinemia?
Hyperinsulinemia is a condition characterized by elevated levels of insulin in the bloodstream.
While insulin is a hormone crucial for regulating blood sugar levels, too much of it can be a problem.
In a healthy individual, insulin helps transport glucose (sugar) from the bloodstream into the body’s cells, where it is used for energy.
However, when insulin levels are consistently high, it may indicate that the body has become resistant to insulin, a condition often associated with obesity.
Many people mistakenly think that hyperinsulinemia is the same as diabetes, but that’s not quite true.
Hyperinsulinemia can occur even before a diabetes diagnosis or without diabetes altogether.
It is a marker of insulin resistance, which often precedes the onset of type 2 diabetes.
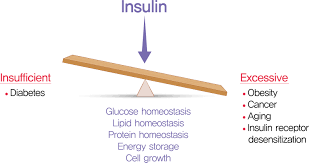
Understanding the Role of Insulin in the Body
Insulin is like the key to your body’s energy vault. Made by the pancreas, this hormone plays a starring role in managing how your body uses and stores fuel—particularly glucose, the sugar that powers your cells.
Here is How it Works:
When you eat a carbohydrate-rich meal (think pasta, bread, or even fruit), your digestive system breaks it down into glucose, which floods into your bloodstream. This rise in blood sugar signals the pancreas to release insulin. Insulin then acts like a key, unlocking your cells so glucose can enter and either be burned for energy or stored for later use.
But the plot thickens when your cells stop responding properly to insulin—a condition known as insulin resistance. Imagine having a stubborn lock that would not turn, no matter how many keys you try. The pancreas, trying to “force the door open,” cranks out more insulin. This leads to hyperinsulinemia, or abnormally high insulin levels in the blood.
What is the Result?
Your body starts storing more fat—especially around the abdomen. Plus, high insulin levels make it harder to break down fat for energy, creating a cycle of weight gain and fatigue. Over time, the pancreas can’t keep up with the demand, and blood sugar levels climb, setting the stage for type 2 diabetes.
A Real-World Example:
Someone who snacks on sugary foods all day might experience constant insulin spikes. Over time, this can lead to insulin resistance—even if they aren’t eating huge quantities. That’s why understanding insulin is crucial—it’s not just about sugar, but how your body handles energy.
How Obesity Leads to Hyperinsulinemia?
Now, how does obesity play a role in this process?
The link between hyperinsulinemia and obesity is well-established in scientific literature. When you’re obese, especially if you have excess abdominal fat, your body becomes more resistant to insulin.
This is largely because fat cells, especially those around the abdomen, release inflammatory substances that interfere with insulin’s ability to do its job effectively.
As a result, your pancreas has to pump out more insulin to keep blood sugar levels in check, leading to hyperinsulinemia.
This excess insulin then signals the body to store more fat, further contributing to obesity.
It is a vicious cycle—obesity leads to insulin resistance, which in turn causes the body to produce more insulin, perpetuating both conditions.
In my case, I noticed that even after adjusting my diet and incorporating exercise, I was not losing as much weight as I expected.
Little did I know that the combination of insulin resistance and hyperinsulinemia was actively working against me, making it nearly impossible to burn fat efficiently.
The Vicious Cycle: Hyperinsulinemia and Weight Gain
One of the biggest frustrations in dealing with both hyperinsulinemia and weight gain is the cycle they create.
When insulin levels are elevated, your body is more likely to store fat rather than burn it.
This is why hyperinsulinemia and weight gain often go hand in hand.
The excess insulin in your bloodstream promotes fat storage, particularly around the abdomen, leading to even more weight gain over time.
What’s more, insulin resistance does not just lead to weight gain; it also makes it harder to lose weight.
High insulin levels signal to your body that it should hold onto its fat stores, making it incredibly challenging to burn fat even when you are trying to lose weight through diet and exercise.
I personally struggled with this for years, thinking I was doing everything right, only to find that my hyperinsulinemia was making it nearly impossible to shed the extra pounds.
My body was in fat-storage mode, thanks to the constant flood of insulin, and it felt like I was fighting an uphill battle.
Scientific Evidence Linking Obesity and Hyperinsulinemia
Several studies have investigated the link between hyperinsulinemia and obesity, highlighting how one fuels the other.
According to a study published in Diabetes Care, individuals with obesity are far more likely to develop hyperinsulinemia due to their increased fat mass and associated insulin resistance.
This study found that hyperinsulinemia was significantly more common in individuals with higher body mass indexes (BMIs), reinforcing the connection between these two conditions.
Another study in the Journal of Clinical Endocrinology & Metabolism pointed out that hyperinsulinemia obesity is not just a consequence but also a driving factor for further weight gain.
It concluded that individuals with elevated insulin levels are more prone to gaining weight, especially in the abdominal area.
These studies show that obesity and hyperinsulinemia are deeply interconnected.
Hyperinsulinemia obesity is not just a temporary phase but a long-term metabolic condition that needs to be managed carefully.
FAQs on Obesity & Hyperinsulinemia
Q-1: What’s the “first push” toward hyperinsulinemia in obesity—resistance or secretion?
A-1: Often both, but many people show insulin hypersecretion early—before glucose rises. Extra calories and circulating free fatty acids (FFAs) amplify β-cell output so fasting and post-meal insulin climb to keep glucose normal. Over time, that compensation can itself encourage insulin resistance, creating a reinforcing loop. So, adopt healthy lifestyle changes to improve your overall health.
Q-2: How do visceral fat and inflammation make the pancreas pump out more insulin?
A-2: Visceral fat drains FFAs and inflammatory signals to the liver and muscle, blunting insulin’s effect there. To overcome this, β-cells release more insulin. Early on, chronic FFA exposure stimulates secretion; if prolonged, it can exhaust β-cells. The early signature is higher basal and meal-stimulated insulin with near-normal glucose.
Q-3: Why can insulin be high even when my glucose looks “fine”?
A-3: The liver normally clears a big share of insulin on first pass. In obesity—especially with fatty liver—hepatic insulin clearance falls, so more insulin escapes into the bloodstream for the same amount secreted. Combine reduced clearance with higher secretion and you get fasting hyperinsulinemia without obvious hyperglycemia.
Q-4: Do gut hormones play a role in obesity-linked hyperinsulinemia?
A-4: Yes. Incretins such as GLP-1 and GIP boost meal-time insulin release. Obesity can alter this incretin effect and gut–brain signaling; in some patterns (frequent refined carbs, larger evening meals), GIP-driven responses remain strong even as tissues resist insulin, sustaining high post-meal insulin levels.
Q-5: Is hyperinsulinemia just a harmless response—or can high insulin worsen weight and resistance?
A-5: Chronic high insulin can favor fat storage and suppress fat breakdown while nudging tissues toward lower sensitivity. So a compensatory rise can become a driver. That’s why strategies that lower insulin demand—modest weight loss, more daily movement, protein-plus-fiber meals, and earlier eating windows—often reduce both insulin levels and resistance.
Q-6: How do researchers tell if high insulin comes from over-secretion or poor clearance?
A-6: They measure insulin and C-peptide (released in equal amounts but not cleared by the liver). If insulin is high but C-peptide isn’t, clearance is likely reduced. If both are high, the pancreas is over-secreting. This split helps target the mechanism—β-cell drive, hepatic clearance, or both.
Takeaway: In obesity, hyperinsulinemia typically reflects a mix of stronger β-cell drive, impaired insulin action, altered incretin signaling, and reduced liver clearance—often appearing while glucose still tests “normal.”
Daily Lifestyle Habits That Contribute to Hyperinsulinemia
Lifestyle habits significantly contribute to obesity and hyperinsulinemia, conditions that reinforce each other, creating a challenging cycle of metabolic disturbances. The interaction between these conditions amplifies their negative impacts, making prevention and management crucial.
Several daily behaviors have been identified as key contributors:
- High-Carb and High-Sugar Diet:
- Consumption of refined carbohydrates and sugars leads to rapid increases in blood sugar levels.
- Excessive insulin release follows, driving fat storage and promoting insulin resistance.
- Frequent consumption of these foods continuously stresses metabolic pathways, exacerbating obesity and hyperinsulinemia.
- Sedentary Lifestyle:
- Lack of physical activity significantly increases central obesity and reduces good cholesterol (HDL), while raising triglycerides and blood glucose.
- Extended periods of inactivity daily elevate the risk of developing metabolic syndrome, further complicating metabolic health.
- Regular exercise, on the other hand, enhances insulin sensitivity and helps manage body weight.
- Chronic Stress:
- Continuous exposure to stress raises cortisol levels, a hormone that directly contributes to elevated blood sugar.
- Persistent high cortisol disrupts normal insulin signaling, promoting insulin resistance.
- Stress-related hormonal imbalances also encourage abdominal fat accumulation, which is closely associated with metabolic disorders.
- Inadequate Sleep:
- Poor sleep hygiene heightens insulin resistance, complicating the body’s ability to manage blood sugar effectively.
- Chronic sleep deprivation has been consistently linked with increased risk of developing metabolic syndrome and obesity.
- Proper sleep patterns help maintain optimal metabolic function and support weight management.
- Circadian Rhythm Disruption:
- Irregular sleep or meal timing disrupts normal glucose metabolism, increasing insulin resistance.
- Maintaining a consistent schedule for sleeping and eating supports overall metabolic health and reduces obesity risks.
- Unhealthy Dietary Fats:
- Diets high in saturated and trans fats negatively impact insulin sensitivity, contributing to metabolic dysfunction.
- Replacing unhealthy fats with unsaturated fats improves insulin responsiveness and promotes overall health.
- Frequent Consumption of Ultra-Processed Foods:
- Ultra-processed foods rich in sugars, unhealthy fats, and additives, stimulate overeating and significantly increase obesity prevalence.
- Prioritizing minimally processed, nutrient-dense foods reduces obesity and improves metabolic health.
To improve metabolic health and reduce the risk of obesity and hyperinsulinemia, it is essential to adopt healthier lifestyle practices including dietary modifications, consistent physical activity, effective stress management, and maintaining adequate sleep and regular routines.
It was not until I started recognizing the lifestyle habits that were working against me that I began to see some progress.
Takeaway
In conclusion, obesity causes hyperinsulinemia by creating an environment where insulin resistance can thrive.
Excess body fat, particularly in the abdominal area, leads to inflammatory responses that interfere with insulin’s ability to regulate blood sugar levels. In response, the body produces even more insulin, which promotes fat storage and leads to further weight gain.
This vicious cycle of hyperinsulinemia and weight gain is one of the main reasons why losing weight can be so difficult for individuals with obesity.
For those of us who have experienced the frustration of trying to lose weight while dealing with hyperinsulinemia, the struggle is real.
High insulin levels make it challenging to burn fat, and the condition itself can promote more weight gain.
But understanding the mechanisms behind hyperinsulinemia obesity is the first step toward breaking that cycle.
References: